Real-Time PCR based on TAQMAN
Molecular biological detection methods have revolutionized medical diagnostics and are an indispensable part of laboratory diagnostics. In many areas of infection diagnostics, the polymerase chain reaction (PCR) has replaced or supplemented the classic detection methods.
Overview

The R-Biopharm RIDA®GENE-Kitsare based on real-time PCR technology for the detection of gastrointestinal, respiratory and nosocomial infections. This highly sensitive and specific method quickly delivers reliable results. A variety of commercial manual or automatic extraction systems can be used to recover the pathogen's DNA or RNA from various sample material. The RIDA®GENE kits can be used on common real-time PCR devices such as the LightCycler®, SmartCycler®, m2000rt (Abbott), Rotor-Gene Q (Qiagen), ABI 7500 and Mx3000P / 3005P. This enables the RIDA®GENE kits to be used flexibly in routine diagnostics.
The R-Biopharm RIDA®GENE kits contain all the components required for the specific detection of pathogens. An included extraction control ensures reliable results. The R-Biopharm RIDA®GENE kits are CE-certified and are evaluated through regular participation in the QCMD (QUALITY CONTROL for MOLECULAR DIAGNOSTICS) round-robin tests.
THE ADVANTAGES OF THE R-BIOPHARM RIDA®GENE KITS:
- High sensitivity
- High specificity
- Contains all the necessary components
- Reliable through extraction control
- Flexible - can be used on all common real-time PCR devices
- Validated - CE certified & QCMD proficiency test participation
Precise instructions, literature and other information on all products are available from us.
New at R-Biopharm
R-Biopharm is now offering the following new products:
RIDA®GENE SARS CoV-2 RBIPG6815
This test is a multiplex real-time RT-PCR for the direct qualitative detection of the novel coronavirus (SARS-CoV-2) RNA from human respiratory samples.
At the end of December, a large number of pneumonia with an unknown cause occurred in Wuhan, a metropolis of China. In early January, a novel coronavirus (SARS-CoV-2) was identified as the cause of these diseases by Chinese authorities. The disease caused by SARS-CoV-2 was officially named COVID-19 ("Corona Virus Disease 2019") and can be transmitted from person to person.
The first cases have also been confirmed in Germany since the end of January 2020. On January 31, 2020, the WHO declared an international health emergency.
Articles in the webshop:
RIDA®GENE ADENOVIRUS RBIPG1005
This test is a real-time PCR for the direct qualitative detection of adenovirus from human throat rinse water, sputum and bronchoalveolar lavage (BAL) and is intended to support the diagnosis of a respiratory infection caused by adenovirus.
Adenoviruses are non-enveloped icosahedral double-stranded DNA (dsDNA) viruses and belong to the Adenoviridae family. They were isolated from human pharyngeal tonsils (adenoids), which is where their name comes from. A distinction is made between 51 human pathogenic adenovirus serotypes, which are divided into six groups (A - F). Adenoviruses cause a number of very different clinical pictures, but in most cases, in addition to ocular and gastrointestinal infections, they are predominantly diseases of the respiratory tract. Children under four years of age are more likely to be affected by the lack of a humoral immune system, but adenoviruses cause respiratory infections in 1–7% of adults. The symptoms of an adenovirus infection range from the common cold to acute bronchitis to pneumonia and, in immunosuppressed patients, also to Acute Respiratory Distress Syndrome (ARDS). Acute respiratory disease is mainly caused by serotypes 1-3, 4, 6, 7, 14 and 21, while serotypes 1-4 and 7 are the most common cause of pneumonia. Some adenovirus serotypes are endemic, with adenovirus outbreaks being described primarily in military facilities. A new variant of serotype 14 led to an outbreak of severe respiratory disease in the USA in 2006/2007 with a mortality rate of 5%. The clinical spectrum of an adenovirus infection also depends, among other things, on its entry point. For example, inhalation of adenovirus 7 results in severe lower respiratory tract disease, while oral ingestion of this serotype results in no or mild infection.
Articles in the webshop:
RIDA®GENE BORDETELLA PERTUSSIS RBIPG2505
Bordetella pertussis is a gram-negative bacterium that causes an acute respiratory infection called pertussis, or whooping cough. Pertussis can cause a serious illness in people of any age, which can be life-threatening, especially in infants. Real-time PCR enables rapid, sensitive and specific detection up to 4 weeks after the onset of symptoms of the disease. In addition, the human pathogenic Bordetella species can be differentiated in a real-time PCR.
RIDA®GENE Bordetella is a real-time multiplex PCR for the direct qualitative detection and differentiation of Bordetella pertussis, Bordetella parapertussis and Bordetella holmesii. After DNA isolation, the gene fragment (IS1001, IS481) specific for Bordetella pertussis, Bordetella parapertussis and Bordetella holmesii is amplified (if available). The assay can be performed on common real-time PCR devices such as Mx3005P, LightCycler® 480II, SmartCycler®, ABI7500, m2000rt, CFX96 or Rotor-Gene Q can be used.
Articles in the webshop:
RIDA®GENE ENTEROVIRUS RBIPG4705
New multiplex real-time RT-PCR for the detection of the most important enteroviruses.
Human enteroviruses comprise a wide range of different pathogens: poliovirus, coxsackievirus A and B, human enterovirus 70/71 and echovirus.
Most enterovirus infections are asymptomatic or cause mild cold-like symptoms. Severe enterovirus infections are e.g. Poliomyelitis, hand, foot and mouth disease, and meningitis. Coxsackieviruses are spread around the world and are the cause of the "summer flu". Other severe infections with coxsackievirus or human enterovirus 70/71 can cause conjunctivitis and myocarditis, while echovirus infections can lead to aseptic meningitis. Echovirus 30 is the most common echovirus serotype that can cause meningitis in Europe, America and Asia.
The new RIDA®GENE enterovirus multiplex real-time RT-PCR allows the simultaneous detection of poliovirus, echovirus, coxsackievirus and human enterovirus 70/71. The RIDA®GENE Enterovirus Test contains all the necessary reagents and delivers results in less than two hours.
Articles in the webshop:


RIDA®GENE PARAINFLUENZA RBIPG5805
Human parainfluenza viruses (HPIV) can cause infections of the upper respiratory and lower respiratory tract.
The four serogroups parainfluenza 1, 2, 3 and 4 are among the most common community-acquired respiratory pathogens worldwide. Parainfluenza virus 1 and 3 infections are common in infants, young children, and immunocompromised and chronically ill patients. In the USA, the annual cost of hospitalization for parainfluenza virus 1 and 3 infections is estimated at $ 186 million, while a croup epidemic caused by parainfluenza virus costs $ 1.30 million.
- Real-time multiplex RT-PCR
- Simultaneous detection and differentiation of hPIV 1, hPIV 3 and hPIV 2/4
- Compatible with the most common real-time PCR devices
- Contains all necessary components, including extraction control (internal control RNA, ICR)
- Delivers results in less than 2 hours
Articles in the webshop:
RIDA®GENE PNEUMOCYSTIS JIROVECII RBIPG1905
Pneumocystis jirovecii (formerly P. carinii) can lead to pulmonary pneumonia and is the most common opportunistic disease in HIV-infected people. Mortality is 100% in patients without treatment and 5-40% in immunocompromised patients who have received treatment. The detection of Pneumocystis jirovecii was previously carried out by immunofluorescence staining. However, due to the low sensitivity, this is replaced by PCR.
The RIDA®GENE Pneumocystis jirovecii kit contains all the components required for real-time PCR and allows 100 determinations in less than 2 hours. The limit of detection is 5 DNA copies per reaction. The assay can be performed on common real-time PCR devices such as Mx3005P, LightCycler® 480II, SmartCycler®, ABI7500, m2000rt, CFX96 or Rotor-Gene Q can be used.
Articles in the webshop:
RIDA®GENE SAPOVIRUS RBIPG1605
This test is a real-time RT-PCR for the direct qualitative detection of sapovirus from human stool samples.
Sapoviruses, also known as Sapporovirus, belong to the Caliciviridae family and are classified as Sapporo-like viruses. Together with the Norwalk-like viruses (norovirus), they are the most common causes of gastroenteritis worldwide. The name sapovirus has its origins in the city of Sapporo in Japan, where the sapovirus was first discovered in a children's orphanage in 1977. While the most common occurrence of sapovirus infections is described in children under the age of five, there are also sapovirus outbreaks in adults. Clinical symptoms of an infection with diarrhea, vomiting and fever are similar to those of a norovirus infection, but a sapovirus infection leads to a much milder form of gastroenteritis.
Since its first discovery in Japan, there have been other outbreaks around the world in countries such as the United States, Canada, Africa, and South Africa. At least five genogroups (GGI - GGV) are described, whereby GGI, GGII, GGIV and GGV can infect humans. To date there have been few epidemiological studies and sapoviruses have rarely been diagnosed due to insensitive methods. Therefore, real-time PCR is a significant advantage in detecting sapovirus infections that cause gastroenteritis.
Articles in the webshop:

RIDA®GENE GASTRO PANEL
“Multiplex” for individualized patient management
Due to the ever increasing demands on routine laboratories, such as the time required for diagnosis and reliable detection of the cause of an infection, the demands on commercially available test systems are also increasing. In order to meet these requirements of today's molecular diagnostics and to offer customers a flexible solution that is tailored to their needs, R-Biopharm AG is introducing its new RIDA®GENE Gastro Panel.
With the RIDA®GENE Gastro Panel it is possible to combine different parameters of the RIDA®GENE real-time PCR tests for each individual patient, which is a cost-effective screening solution for the diagnostic laboratory.
For more information on the respective RT PCR kits, visit our webshop, contact us directly using our contact form or send us an email to mail@szabo-scandic.com.
- UNIQUE
- Design your individual panel
- COST EFFICIENT
- Simultaneous detection of up to 18 gastrointestinal pathogens
- Panel combination adapted to the patient
- FAST
- Screening within 3 hours
Viruses |
Bacteria |
Protozoa |
| NorovirusGI/GII | Salmonella spp. | Giardia lamblia |
| Rotavirus A | Campylobacter spp. | Cryptosporidium spp. |
| Adenovirus | Yersinia enterocolitica | Entamoeba histolytica |
| Astrovirus | Shiga-like toxin-producing E. coli (STEC) stx1/stx2 | Dientamoeba fragilis |
| Sapovirus | Enteropathogenic E. coli (EPEC) Enteroaggregative E. coli (EAEC) Enterotoxigenic E. coli (ETEC) lt/st Shigella spp./Enteroinvasive E. coli (EIEC) Clostridium difficile tcdA/tcdB |
The following ready-made multiplex panels for gastrointestinal and nosocomial infections from R-Biopharm:
Viral infections::
Bacterial infections:
Parasitic Infections:
Nosocomial infections:
R-Biopharm also has a wide range of Singleplex RT-PCR kits in various areas as well as additional products for color compensation and extraction.
RIDA®GENE CYCLERLIST
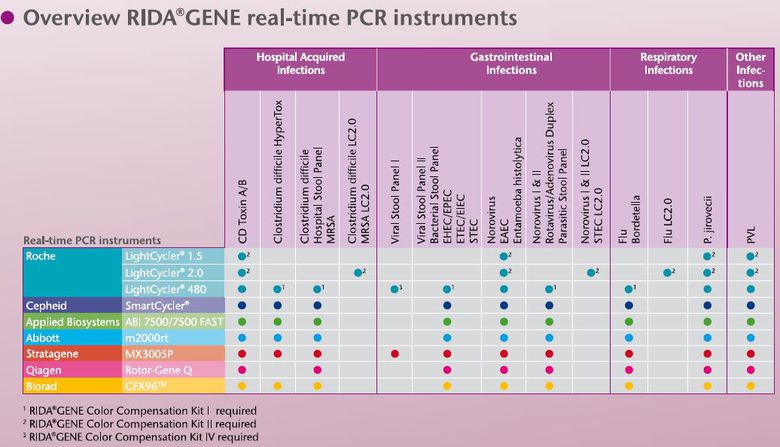
For the complete range of R-Biopharm products, please visit our webshop.

Clonit has some interesting and unusual real-time PCR kits based on TaqMan probes. The analysis can be carried out with the most common real-time PCR analyzers. All kits contain ready-to-use reagents.
Selection of some genetic diseases (qualitative evidence, wild type and mutant genotype included):
Interleukin 28B
Markers of interferon resistance in the treatment of hepatitis C infections.
IL-28B Kit is a test for allelic delimitation of rs8099917 and rs12979860 polymorphisms, which are a relevant prognostic factor for successful therapy with interferon (lambda 3) in HCV-positive patients.
FSH receptor
Genotyping of A919 / thr307ala and A2030G / Asn680Ser.
Celiac disease
Multiple detection of 5 haplotypes that are predictive for this disease. Genotyping of HLADQ2 and HLADQ8.
In development: lactose intolerance (G13907A mutation)
Selection of some infectious diseases (quantitative / qualitative detection, calibration curve and positive control included):
Fecal Parasites
Multiple detection of Entamoeba histolytica, Criptosporidium parvum and Giardia Lamblia.
Malaria Panel
Multiple detection and identification of P. falciparum, P. malariae, P. vivax and P. ovale
In development: JCV enterovirus, varicella zoster virus, adenovirus
Precise instructions, literature and other information on all products are available from us.

Our partner Clonit specializes in end-point (single or nested) PCR products.
The new FAST-Taq PCR kits/reagents and Multiplex FAST PCR kits/reagents for the detection of bacteria, viruses and parasites using pre-made gels (EtBr-free) offer the following advantages:
- A single DNA / RNA extraction of a sample for multiplex products
- Uniform amplification protocol with a unique master mix
- One-step gel separation
- Simply pipette the amplicon into the slot of the gel
- No further reagents required
- "FAST" procedure
- From extraction to result in less than an hour
- Increased sensitivity and reliability
- Reduced manual work steps
- No toxic or hazardous reagents
- Inexpensive: up to 5 results at once with multiplex products
Available products:
Viruses: HCV, HBV, HDV, HIV, HHV-6, HPV-Screening, HPV-11, HPV-16, HPV-18, HPV-33, Parvovirus B19, Poliovirus, CMV, JCV, HSV 1 and II, EBV, VZV
Bacteria: Helicobacter pylori, Mycobacterium tuberculosis, Chlamydia trachomatis, Chlamydophila pneumoniea, Mycoplasma pneumoniae, Legionella pneumophila, Borrelia burgdorferi, Neisseria gonorrhoeae, Ureoplasma urealyticum, Clostridium sp
Parasites/protozoa: Toxoplasma gondii, Plasmodium Screening, Plasmodium falciparum, Plasmodium ovale, Plasmodium vivale, Plasmodium malariae, Leismania spp.
Genetic parameters: Factor V Leiden, Factor II, MTHFR (G20210A), MTHFR (A1298C), HLA-B27
Multiplex FAST PCR kits simultaneously detect different pathogens in the same sample amplification.
Multiplex STD Panel (Sexually Transmitted Diseases)
Chlamydia trachomatis, Neisseria gonorrhoeae, Ureoplasma urealyticum, Mycoplasma hominis, Mycoplasma genitalium
Multiplex HPV screening and high risk detection
Simultaneous differentiation of the L1 gene for screening purposes and of the oncogenic region E6 / E7 for high / low risk detection.
Multiplex Atypical Bacteria Pneumoniae
Chlamydophila pneumoniae, Mycoplasma pneumoniae, Legionella pneumophila
Multiplex fecal enterocolitis bacteria 1
Shigella spp, Salmonella spp, Cambylobacter spp
Multiplex fecal enterocolitis bacteria 2
E. coli O157: H7, Yesinia enterocolitica, Clostridium difficile
Multiplex Fecal Parasite Panel:
Cryptosporidium parvum, Entamoeba histolytica, Giardia lamblia
Multiplex periodontal diseases:
Actinobacillus actinomycetemcomitans, Prevotelle intermedia, Porhyromonas gingivalis
Precise instructions, literature and other information on all products are available from us.
CLONITE ELECTROPHORESIS SYSTEM

Clonpact Clongel System
CLONPACT
An innovative system for DNA and RNA separations using gel electrophoresis with an integrated electrophoresis chamber, electrical supply and UV illuminator.
CLONGEL
Ready-to-use, pre-made agarose gel, ethidium bromide-free in an 11-slot format..

CLONIT CAMERA SYSTEM
USB camera system with software for the detection of electrophoresis patterns as well as image and data storage.
Benefits:
- User friendly
- Complete system
- No gel manipulation
- Direct transfer of the electrophoresis samples to the PC in real time
- Fast
- 10 minutes to the results and data storage
- Safety
- Agarose gel without EtBr
- Electrophoresis without touching the gel
- Addition of additional reagents is not necessary
- Inexpensive
- Similar costs to traditional agarose gel preparation
Precise instructions, literature and other information on all products are available from us.
For the complete range of Clonit products, please visit our webshop.
 SavvygenTM are characterized by lyophilized ready-to-use mastermix in Stripwell format, which enables transport and storage at room temperature, except for the STI panels, which have to be transported with dry ice. In addition to respiratory and gastrointestinal infections, sexually transmitted infections are also detected.
SavvygenTM are characterized by lyophilized ready-to-use mastermix in Stripwell format, which enables transport and storage at room temperature, except for the STI panels, which have to be transported with dry ice. In addition to respiratory and gastrointestinal infections, sexually transmitted infections are also detected.
For the complete range of Savyon Diagnostics products, please visit our webshop.

Liferiver products cover over 300 target sequences. In addition to the classic viral and bacterial palette, sexually transmitted diseases and specific issues are also covered. The small test format (25 reactions) enables the reagents to be used up completely, even if a parameter is rarely asked for.
Advantages of the real-time PCR kits:
- High sensitivity
- High specificity
- Ready-to-use reagents
- Integrated extraction control
- Flexible - can be used on common real-time PCR devices
- CE-IVD
- Shelf life up to 2 years
For the complete range of Liferiver products, please visit our webshop.

 Deutsch
Deutsch


