Szabo-Scandic: Study of research trends in Austria
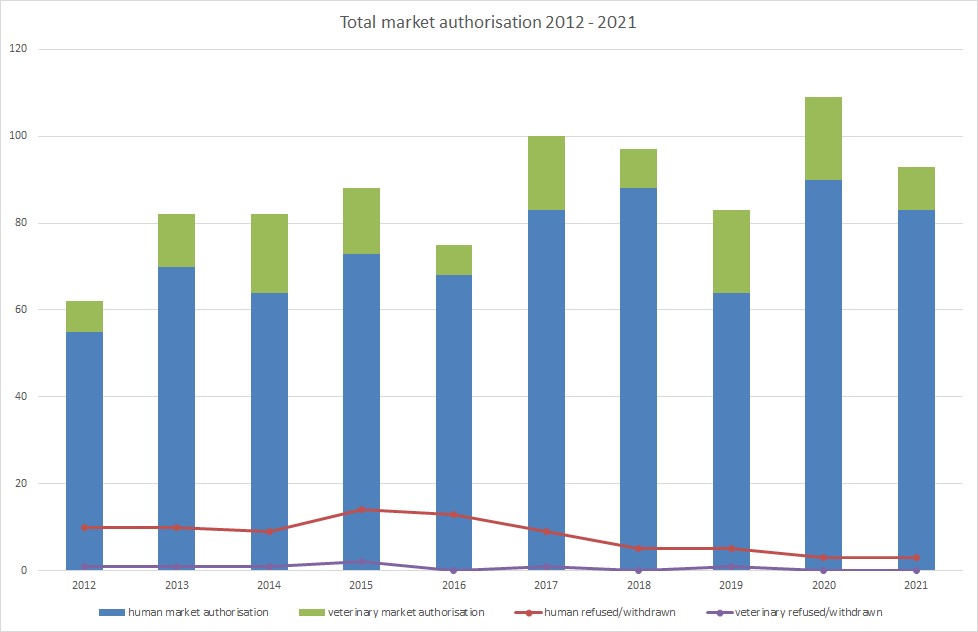
Vienna, January 2022: A total of 83 drugs for human consumption and 10 for veterinarian use were approved for sale in the EU market in 2021. In the previous year, 2020, 90 human and 19 veterinarian drugs were approved. This displays a decline of 15%. Admittedly, 2020 was an exceptional year with a great number of approvals. Most therapies were, just as the years before, registered for the discipline oncology. Additionally, private, and public research institutes expected numerous publications about vaccines and drugs to treat infectious diseases. Indeed, in the past two years, more vaccines were approved than in the last ten years. This is revealed by the research barometer, published recently by the medical product specialist Szabo-Scandic.
To analyze research trends in Austria, Szabo-Scandic reviewed the market approvals of drugs by the EMA, the European Medicines Agency, and compared the data with the assessment of 81 specialists from research institutes, such as universities, laboratories, hospitals, or manufacturers.
In 2021 noticeably less drugs were admitted to the EU market compared to 2020. However, approvals for human medicines were above average compared to the last 10 years and rank in 4th place with 93 admissions. Since 2012, the highest number of admissions were recorded in 2020. With a total of 109 market approvals for human medicinal and veterinary medical therapies, 2020 is first in the ranking, followed by 2016 with 100 admissions and 2018 with 97 admissions. The majority of admissions were for drugs for human use, the market for veterinarian therapies is distinctly less dynamic.
Influence by COVID-19
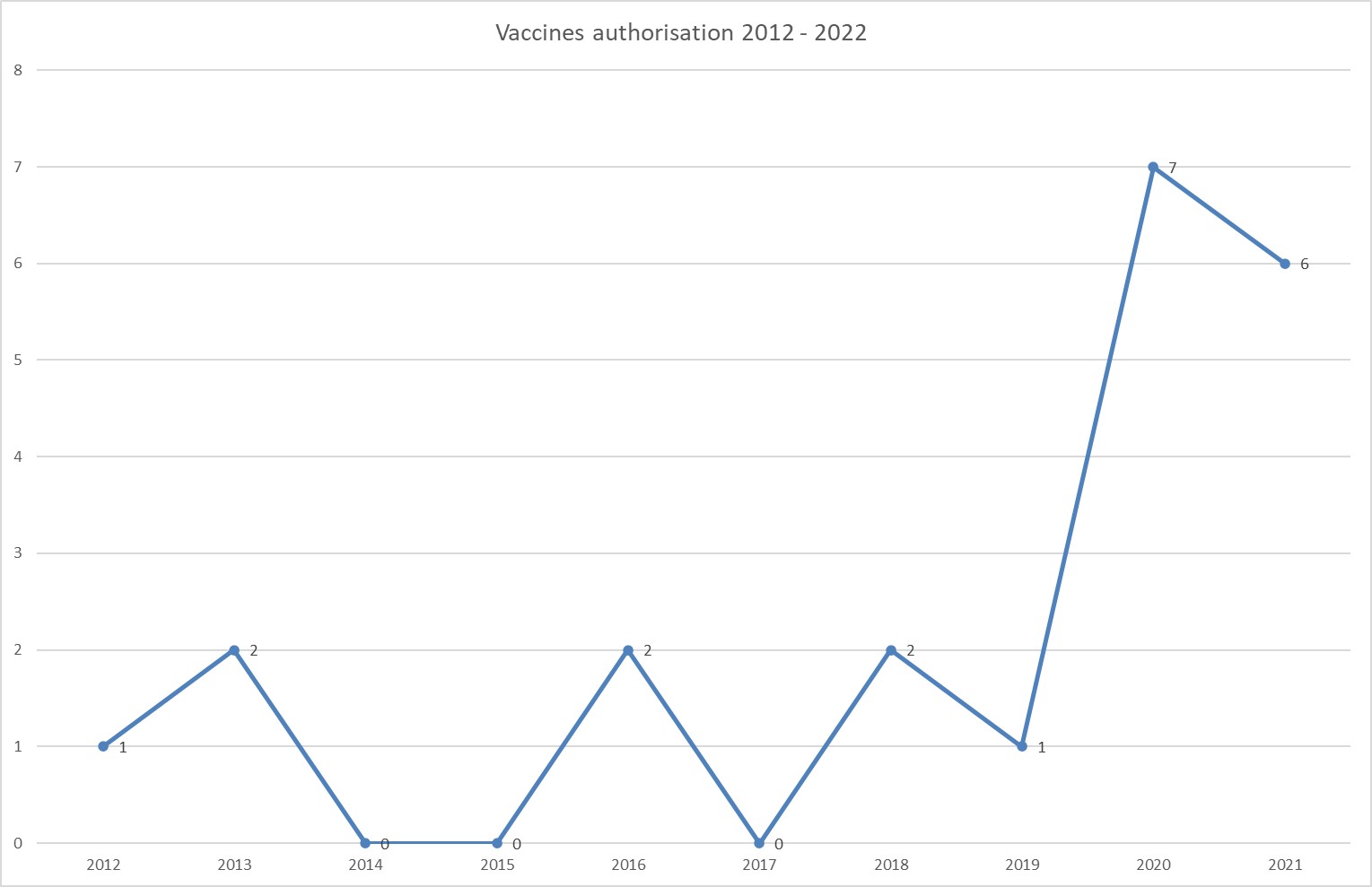
The coronavirus pandemic particularly affected the development of vaccines. Not only were COVID-19 vaccines – manufactured by BioNTech Pfizer, Moderna, AstraZeneca and Janssen – approved in 2020 and 2021, the vaccine Novavax was also approved in December 2021 for the European market. There are also new vaccines for Ebola, cholera, meningococci, and influenza. In 2021, a total of six vaccines were admitted – of which four were to prevent COVID-19. In 2020, 7 vaccines achieved market maturity, inclusive of the first COVID-19 vaccine.
Research focus 2021
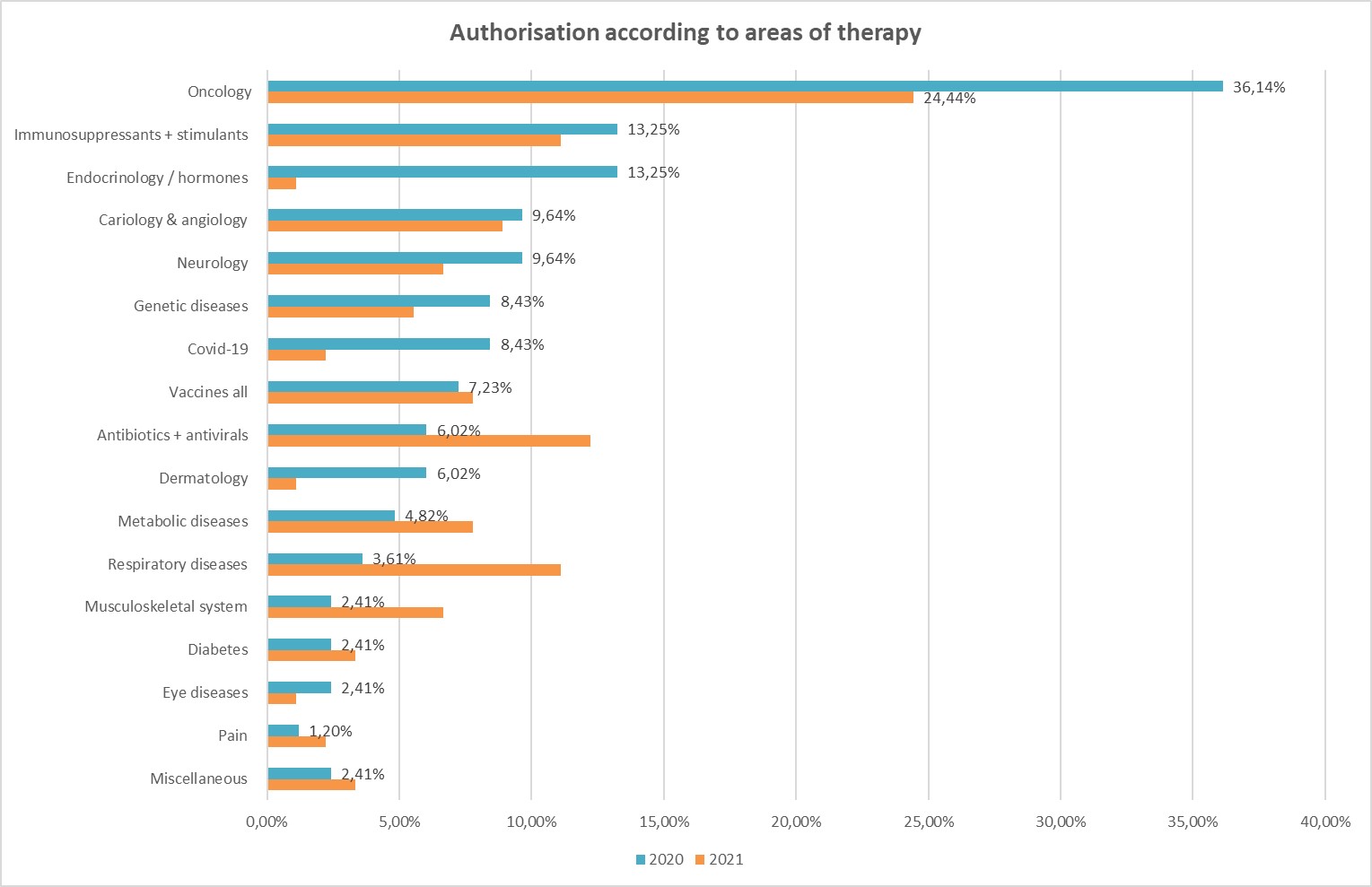
Most drugs were launched on the EU market against oncological diseases in both 2020 and 2021. 24.4% of all drugs approved in 2020 were designed for use in cancer therapies, rising to 36.1% in 2021. Drugs against COVID-19 ranked 7th in 2021. However, only four drugs have been approved for the treatment of corona diseases to date. Three received the green light for marketing by the EMA in 2021. One drug received a secondary use in 2020 with approval for COVID-19 therapy. The rapid development of vaccines against COVID-19 is due to the use of platform vaccines (mRNA and vector), as they could build on already developed "blueprints," and to rolling reviews – which were created explicitly for this purpose - during which regulatory authorities were involved at an early stage. At the same time, the development of therapies against coronavirus infections clearly tied up visible resources. In 2021, approvals of drugs for respiratory diseases as well as antivirals and antibiotics declined significantly.
Research focus on vaccines and infectious diseases
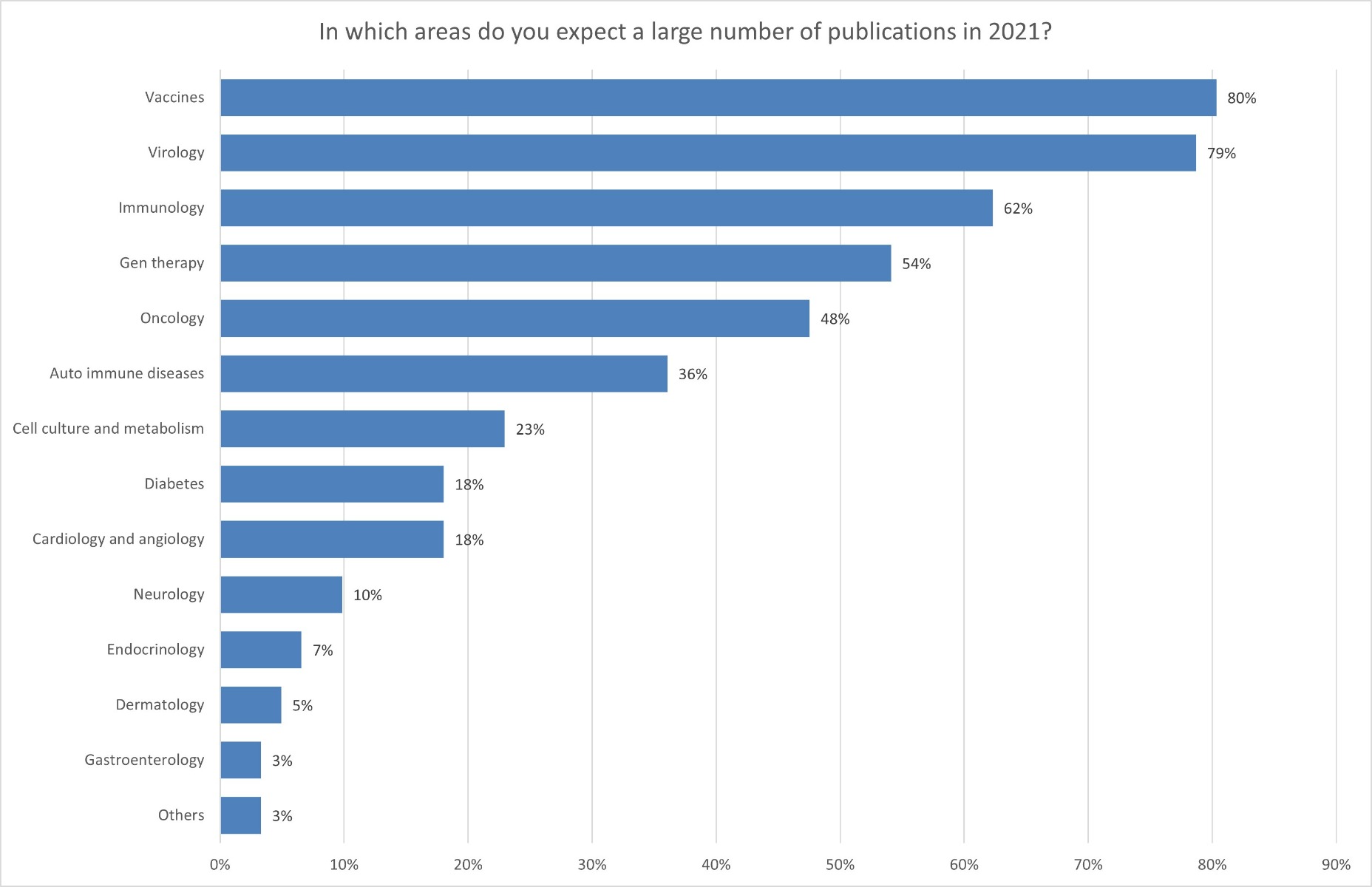
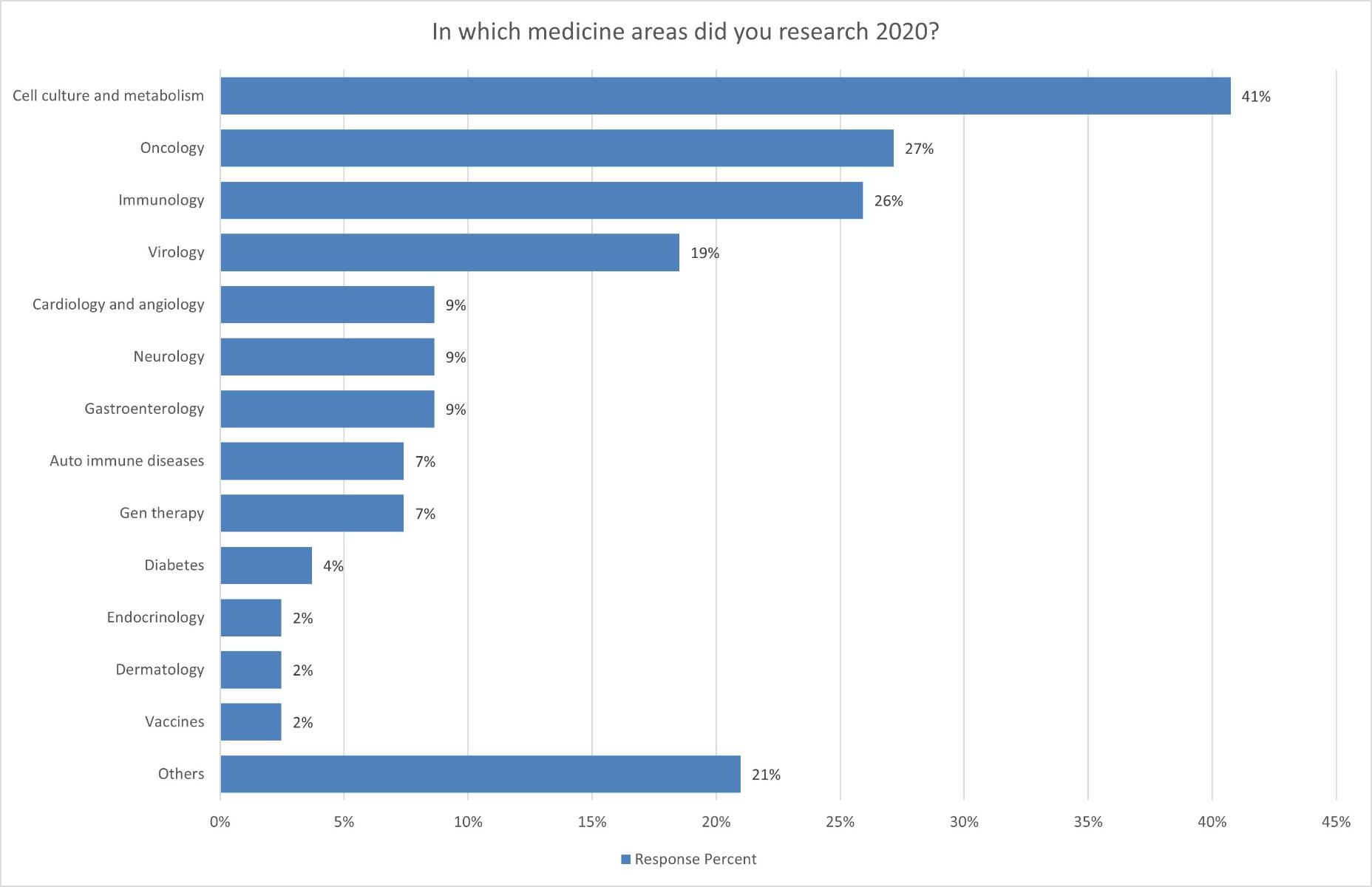
This development is also consistent with the statements made by the research institutions in Austria. In 2021, according to the institutions, research was increasingly focused on infectious diseases and the development of new vaccines in view of the coronavirus pandemic. In 2020, most respondents were still conducting research on cell culture and cell metabolism (41%), followed by oncology (27%). Nevertheless, in 2021, researchers expected most publications to be in the area of vaccine development (80%), closely followed by virology (79%) and immunology (62%). This is a clear signal that the research community is currently heavily engaged in combating COVID-19. Other research areas have been and are still being put on the back burner.

 Deutsch
Deutsch